Chapter:- Elements, compounds and mixture pg:- 18
Principles of Distillation:
Boiling Point: Each liquid in a mixture has a specific temperature at which it transitions from the liquid phase to the vapor phase, known as its boiling point. Distillation relies on the differences in boiling points to separate components.
Volatility: The volatility of a substance is a measure of its tendency to vaporize. In a mixture, the more volatile components (those with lower boiling points) will vaporize first.
Condensation: After vaporization, the vapor can be cooled to convert it back into a liquid (condensation). This process allows for the collection of purified liquid.
Types of Distillation:
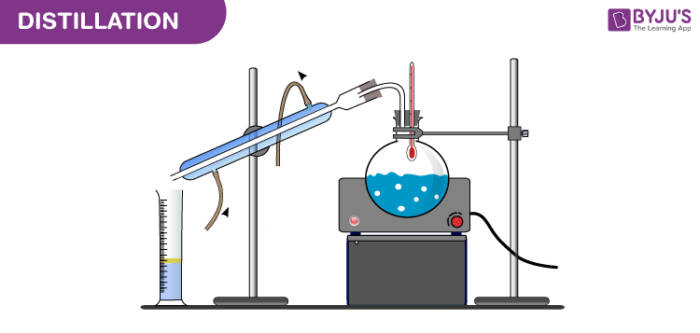
1. Simple Distillation
Principle: Simple distillation separates components based on significant differences in boiling points. It's ideal when the boiling points differ by at least 25°C.
Apparatus Configuration:
Distillation Flask: Holds the liquid mixture.
Heat Source: Heats the mixture to the boiling point of the more volatile component.
Thermometer: Measures the temperature of the vapor.
Condenser: Cools the vapor back into liquid form.
Receiving Flask: Collects the distilled liquid.
Procedure:
Heat the mixture until the component with the lower boiling point vaporizes.
The vapor passes through the condenser.
The vapor condenses back to liquid and is collected in the receiving flask.
Applications and Examples:
Purification of Drinking Water: Removing impurities from water.
Distillation of Alcohol: Separating alcohol from fermentation mixtures.
2. Fractional Distillation
Principle: Fractional distillation is used for separating mixtures of liquids with closer boiling points (less than 25°C difference). The process involves multiple condensation and vaporization cycles.
Apparatus Configuration:
Distillation Flask: Contains the liquid mixture.
Fractionating Column: Filled with packing material to provide a large surface area for repeated vaporization-condensation cycles.
Thermometer: Measures the vapor temperature.
Condenser: Cools the vapor.
Receiving Flask: Collects the distillate.
Procedure:
Heat the mixture until the components vaporize.
Vapor rises through the fractionating column, where it undergoes repeated condensation and vaporization.
Components with lower boiling points reach the top of the column and enter the condenser.
Condensed vapor is collected as distillate.
Applications and Examples:
Petroleum Refining: Separating crude oil into fractions like gasoline, kerosene, and diesel.
Production of High-Purity Chemicals: Separating chemical mixtures in industrial processes.
3. Vacuum Distillation
Principle: Vacuum distillation reduces the pressure above the liquid, lowering the boiling points of the components. This is particularly useful for substances that decompose at high temperatures.
Apparatus Configuration:
Distillation Flask: Contains the mixture.
Vacuum Pump: Reduces the pressure inside the apparatus.
Thermometer: Monitors the temperature.
Condenser: Cools the vapor.
Receiving Flask: Collects the distillate.
Procedure:
Apply a vacuum to lower the pressure in the system.
Heat the mixture until the components vaporize at lower temperatures.
The vapor passes through the condenser and is collected as distillate.
Applications and Examples:
Distillation of Heat-Sensitive Compounds: Such as high-boiling organic compounds and oils.
Pharmaceutical Industry: Producing high-purity active ingredients.
4. Steam Distillation
Principle: Steam distillation involves passing steam through a mixture to vaporize components that are immiscible with water. The combined vapor of water and the volatile compounds is then condensed.
Apparatus Configuration:
Distillation Flask: Contains the mixture.
Steam Generator: Produces steam that is passed through the mixture.
Thermometer: Measures the temperature.
Condenser: Cools the vapor.
Receiving Flask: Collects the distillate.
Procedure:
Introduce steam into the distillation flask containing the mixture.
Volatile components vaporize with the steam.
The vapor is condensed and collected in the receiving flask.
The mixture of water and volatile components is separated.
Applications and Examples:
Extraction of Essential Oils: From plant materials like eucalyptus, lavender, and citrus peel.
Production of Aromatics: In the fragrance and flavor industries.
5. Azeotropic Distillation
Principle: Azeotropic distillation is used to break azeotropes (mixtures that distill without changing composition). This method often involves adding another component to create a new azeotrope with a different boiling point.
Apparatus Configuration:
Distillation Flask: Contains the azeotropic mixture and the entrainer.
Thermometer: Monitors the vapor temperature.
Condenser: Cools the vapor.
Receiving Flask: Collects the distillate.
Procedure:
Add an entrainer to the azeotropic mixture.
Heat the mixture until the new azeotrope forms and vaporizes.
The vapor is condensed and collected.
Separate the components of the new azeotrope.
Applications and Examples:
Separation of Ethanol and Water: Using benzene or cyclohexane as an entrainer.
Chemical Industry: Breaking azeotropes in the production of high-purity solvents.
6. Destructive Distillation
Principle: Destructive distillation involves heating organic materials in the absence of air to decompose them into simpler compounds.
Apparatus Configuration:
Retort or Distillation Flask: Contains the organic material.
Heat Source: Heats the material.
Condenser: Cools the vapor.
Receiving Flask: Collects the distillate.
Procedure:
Heat the organic material in the absence of air.
Decomposition produces volatile compounds.
The vapor is condensed and collected.
Applications and Examples:
Production of Coal Tar: From coal.
Charcoal Production: From wood.
Components of a Distillation Apparatus:
Distillation Flask: The container in which the mixture to be distilled is placed and heated.
Heat Source: Provides the necessary energy to vaporize the more volatile components. Common heat sources include Bunsen burners, electric heating mantles, or hot plates.
Fractionating Column (in fractional distillation): A vertical column packed with materials (e.g., glass beads) that provide a large surface area for repeated condensation and vaporization, enhancing separation.
Condenser: Cools the vapor back into a liquid. Common types include water-cooled condensers (Liebig condensers) and air-cooled condensers.
Receiving Flask: Collects the distilled liquid (distillate).
Thermometer: Monitors the temperature of the vapor, ensuring precise control of the distillation process.