Page Number - 64-65
Chapter - 3
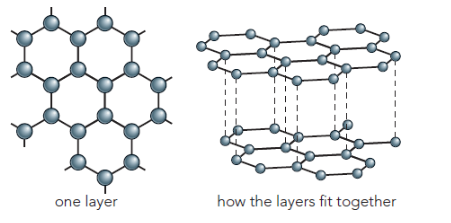
Graphite is a different form of carbon that does conduct electricity . The carbon atoms are arranged in a different way in the molecular structure of graphite. They are arranged in flat layers of linked hexagons
Each graphite layer is a two-dimensional giant molecule
Each carbon atom is bonded to three others by strong covalent bonds
Between the layers there are weaker forces of attraction
The layers are able to slide over each other easily. This means that graphite feels slippery and can be used as a lubricant
The most distinctive property, however, results from the fact that there are free electrons not used for covalent bonding by the atoms in the layers. These electrons can move between the layers, carrying charge, so that graphite can conduct electricity in a similar way to metals.


Why graphite cant't conduct electricity like carbon?
In last sentence you mentioned that graphite can conduct electricity Why?
Describe the atomic structure of graphite and how it differs from diamond.
In 5th point - graphite can use as lubricant. graphite is a solid material then how can it act as a lubricant?
Metallic bonding is mentioned in title but nothing in post why?