Chapter- elements, compounds and mixtures pg:- 17
Crystallization is a process by which a solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. It is a fundamental process used in chemistry and materials science for purifying substances and creating materials with specific properties.
Basic Principles of Crystallization:
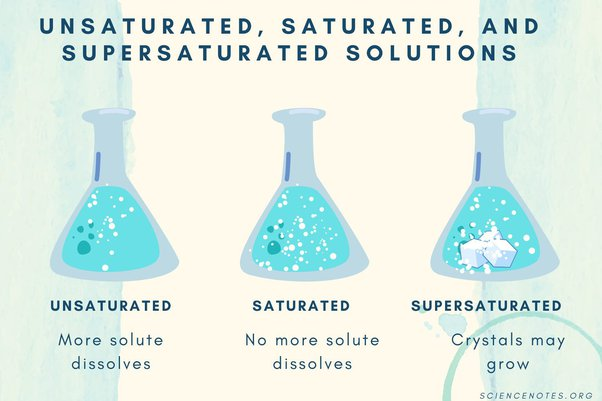
Supersaturation: Crystallization typically occurs from a solution. The solution must be supersaturated, meaning it contains more dissolved material than it can normally hold at a given temperature. Supersaturation is the driving force for crystallization.
Nucleation: The process begins with nucleation, where small clusters of atoms or molecules form. Nucleation can be:
Homogeneous: Occurring uniformly throughout the solution.
Heterogeneous: Occurring on surfaces or impurities present in the solution.
Crystal Growth: Once nucleation occurs, the crystal grows as more atoms or molecules join the lattice. The rate of growth depends on factors such as temperature, concentration, and the nature of the solvent and solute.
Steps in the Crystallization Process:
1.Preparation of the Solution: A solution is prepared by dissolving the solute in a solvent, often at an elevated temperature to increase solubility.
2.Supersaturation: The solution is then cooled or the solvent is evaporated to reach a supersaturated state. In cooling crystallization, the temperature is lowered to reduce solubility. In evaporative crystallization, the solvent is slowly evaporated.
3.Nucleation: Initial small crystals (nuclei) form. The rate of nucleation can be controlled by changing the level of supersaturation, temperature, and agitation of the solution.
4.Crystal Growth: Nuclei grow into larger crystals as additional molecules attach to the existing crystal lattice. The conditions (temperature, concentration, and impurities) must be carefully controlled to obtain crystals of desired size and quality.
5.Harvesting Crystals: Once the crystals have reached the desired size, they are separated from the remaining solution (mother liquor). This can be done by filtration or centrifugation.
6.Drying: The crystals are dried to remove any adhering solvent. This can be done using air drying, vacuum drying, or other methods.
Types of Crystallization:
Cooling Crystallization: This method involves cooling a hot, saturated solution to induce crystallization. As the temperature decreases, the solubility of the solute drops, leading to the formation of crystals.
Evaporative Crystallization: In this method, the solvent is slowly evaporated from the solution, increasing the concentration of the solute until crystals form.
Precipitation Crystallization: This occurs when a chemical reaction in the solution produces a compound that has low solubility, leading to its crystallization.
Reactive Crystallization: Crystals form as a result of a reaction between solutes in a solution. This is often used in chemical synthesis and pharmaceuticals.
Applications of Crystallization:
Purification: Crystallization is widely used to purify compounds in the chemical and pharmaceutical industries. Impurities are excluded from the crystal lattice, resulting in highly pure products.
Material Science: Crystallization is crucial in producing materials with specific properties, such as semiconductors and optical materials. The properties of a crystal can be tailored by controlling the crystallization process.
Food Industry: Crystallization processes are used in the production of sugar, salt, and other crystalline food products.
Pharmaceuticals: The crystallization of active pharmaceutical ingredients (APIs) is essential for ensuring the correct dosage and bioavailability of medications.
Factors Affecting Crystallization:
Temperature: Temperature affects solubility and the rate of nucleation and growth. Precise control of temperature is crucial for obtaining crystals of the desired size and quality.
Concentration: The degree of supersaturation influences the nucleation and growth rates. A higher degree of supersaturation generally leads to faster nucleation but smaller crystals.
Solvent: The choice of solvent affects the solubility of the solute and the properties of the resulting crystals.
Impurities: Impurities can either inhibit or promote nucleation and growth, affecting the purity and morphology of the crystals.
Agitation: Stirring or shaking the solution can influence the rate of nucleation and crystal growth, as well as the size distribution of the crystals.