Introduction
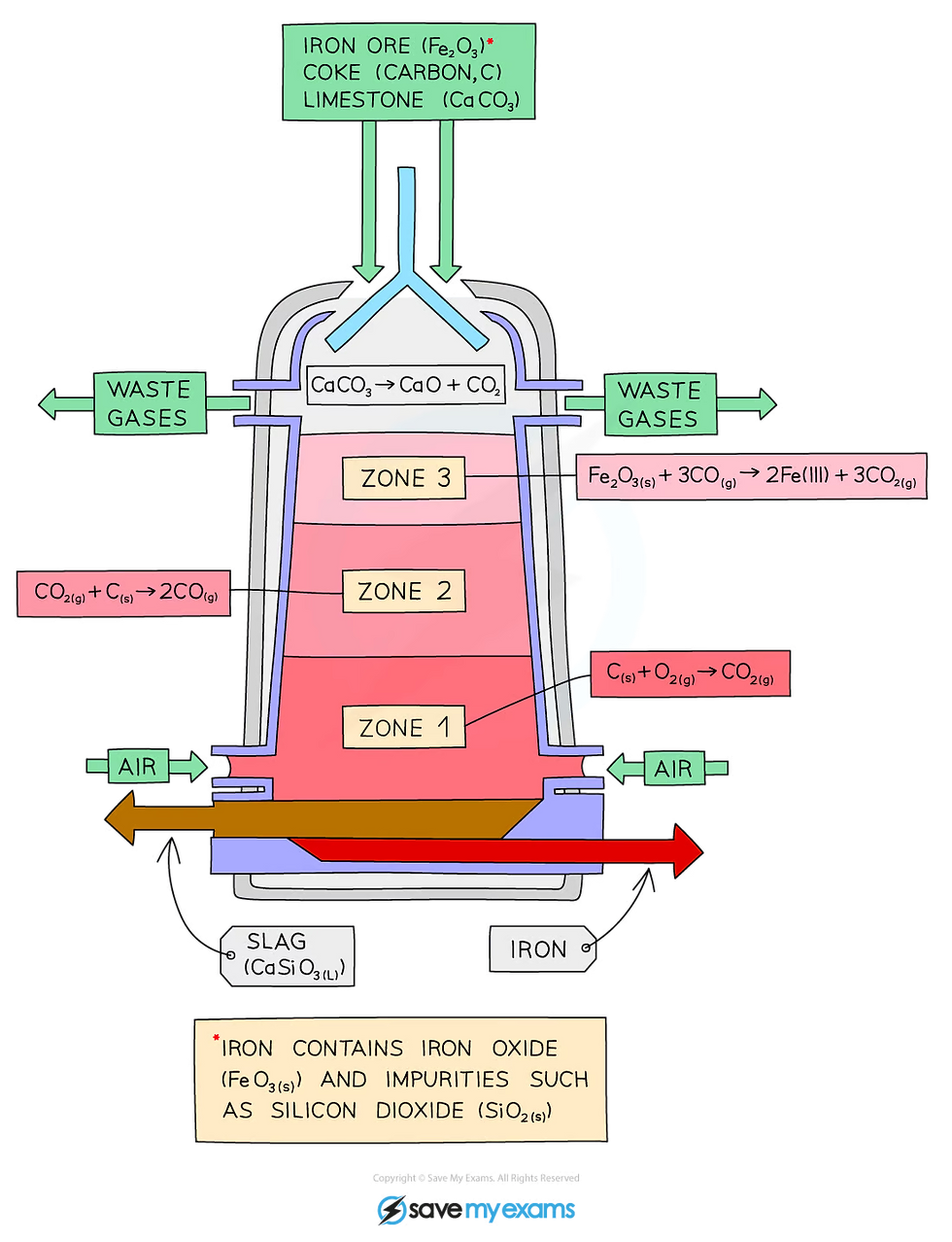
Iron is extracted in a large container called a blast furnace from its ore, hematite
Modern blast furnaces produce approximately 10,000 tonnes of iron per day
The blast furnace
The raw materials: iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace
Hot air is blown into the bottom
Zone 1
Coke burns in the hot air forming carbon dioxide
The reaction is exothermic so it gives off heat, heating the furnace
carbon + oxygen → carbon dioxide
Zone 2
At the high temperatures in the furnace, more coke reacts with carbon dioxide forming carbon monoxide
Carbon dioxide has been reduced to carbon monoxide
carbon + carbon dioxide → carbon monoxide
Zone 3
Carbon monoxide reduces the iron(III) oxide in the iron ore to form iron
This will melt and collect at the bottom of the furnace, where it is tapped off
iron(III) oxide + carbon monoxide → iron + carbon dioxide
Limestone (calcium carbonate) is added to the furnace to remove impurities in the ore
The calcium carbonate in the limestone thermally decomposes to form calcium oxide
calcium carbonate → calcium oxide + carbon dioxide
The calcium oxide formed reacts with the silicon dioxide, which is an impurity in the iron ore, to form calcium silicate
This melts and collects as a molten slag floating on top of the molten iron, which is tapped off separately
calcium oxide + silicon dioxide → calcium silicate
Zone 1
The burning of carbon (coke) to provide heat and produce carbon dioxide:
C (s) + O2 (g) → CO2 (g)
Zone 2
The reduction of carbon dioxide to carbon monoxide:
CO2 (g) + C (s) → 2CO (g)
Zone 3
The reduction of iron(III) oxide by carbon monoxide:
Fe2O3 (s) + 3CO (g) → 2Fe (I) + 3CO2 (g)
The thermal decomposition of calcium carbonate (limestone) to produce calcium oxide:
CaCO3 (s) → CaO (s) + CO2 (g)
The formation of slag:
CaO (s) + SiO2 (s) → CaSiO3 (l)






What are the main products and by-products of the blast furnace process?
How is the iron extracted from the blast furnace further processed to make steel?
What environmental impacts are associated with the extraction of iron?
How does the reactivity series influence the method used to extract iron?
What are some modern advancements in the extraction of iron that aim to reduce environmental impact?