Titration calculations #chemistry
Titrations are a method of analysing the concentration of solutions
Acid-base titrations are one of the most important kinds of titrations
Titrations can determine exactly how much alkali is needed to neutralise a quantity of acid – and vice versa
You may be asked to calculate:
The moles present in a given amount
The concentration or volume required to neutralise an acid or a base
Once a titration is completed and the average titre has been calculated, you can calculate the unknown variable using the formula triangle as shown below
A solution of 25.0 cm3 of hydrochloric acid was titrated against a solution of 0.100 mol/dm3 NaOH.
12.1 cm3 of NaOH was required for a complete reaction.
Determine the concentration of the acid.
Answer:
Step 1: Write the equation for the reaction:
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Step 2: Calculate the number of moles of the NaOH
Moles = () x concentration
Moles of NaOH = 0.012 dm3 x 0.100 mol / dm3 = 1.21 x 10–3 mol
Step 3: Deduce the number of moles of the acid
Since the acid reacts in a 1:1 ratio with the alkali, the number of moles of HCl is also 1.21 x 10–3 mol
This is present in 25.0 cm3 of the solution (25.0 cm3 = 0.025 dm3)
Step 4: Find the concentration of the acid
0.0484 mol/dm3










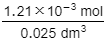
1. How do variations in the concentration of the titrant and analyte affect the accuracy and precision of titration results?
2 .In what ways can titration techniques be adapted or modified to analyze complex mixtures or solutions with multiple acidic or basic components?
3. How do environmental factors, such as temperature and humidity, influence the outcomes of titration experiments, and how can these factors be controlled or accounted for?
4. What are the ethical considerations involved in conducting titration experiments, particularly when using potentially hazardous chemicals, and how can safety protocols be improved or enhanced?
5. How might titration calculations be applied in real-world scenarios beyond the laboratory, such as in environmental monitoring, pharmaceutical quality control, or food safety testing?